-
Publish Your Research/Review Articles in our High Quality Journal for just USD $99*+Taxes( *T&C Apply)
Offer Ends On
Naoko Abe, Shoji Oura* and Shinichiro Makimoto
Corresponding Author: Shoji Oura, Kishiwada Tokushukai Hospital, 4-27-1, Kamori-cho, Kishiwada-city, OSAKA, 596-8522, Japan
Received: June 09, 2022 ; Revised: June 27, 2022 ; Accepted: June 30, 2022
Citation: Abe N, Oura S & Makimoto S. (2022) Feasible Nipple Base Resection for Breast Cancer Located Close to the Nipple-Areolar Complex. J Can Sci Res Ther, 1(2): 1-5.
Copyrights: ©2022 Abe N, Oura S & Makimoto S. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Views & Citations
Likes & Shares
Abstract
An 84-year-old woman with diabetes mellitus (DM) developed breast cancer in the inner and lower quadrant of her right breast. Both mammography and ultrasound showed a well-defined mass 4.8cm in size. Plain computed tomography (CT) showed that the tumor located very close to the nipple-areolar complex. The presence of pacemaker in her chest wall and her impaired renal function made us difficult to preoperatively evaluate the cancer distribution in the breast with precise images, i.e., magnetic resonance imaging or enhanced CT. Based on the strong request of the patient, we tried to do partial mastectomy with nipple preservation to the patient. Our surgical techniques were as follows; 1. Skin flap just around the nipple-areolar complex was made with the preservation of certain amount of subcutaneous fat to possibly keep the venous return from the nipple-areolar complex. 2. When the flap was almost made to the nipple base, we bluntly dissected the nipple base just between the mammary gland and the subcutaneous fat with a curved Mosquito forceps until sub-nipple mammary gland was completely detached from the surrounding fat tissue. 3. We put one blade of the Mayo scissors into the dissected space under the sub-nipple mammary gland, then moved the scissors themselves toward the nipple base as close as possible with the blades being kept open, and lastly cut the sub-nipple mammary gland under adequate counter traction by pulling the mammary gland for the opposite direction of the nipple. After confirming no macroscopic mammary gland left on the nipple base, frozen section for pathological marginal evaluation was done to the stump of the sub-nipple mammary gland. No cancer infiltrates to the nipple base and other surgical margins led us to directly close the partial mastectomy cavity without drain insertion. The patient was discharged from the hospital without any events. The patient, however, later developed DM-induced surgical site infection but eventually healed without no ischemic change of the nipple-areolar complex. Our nipple base resection techniques should be able to provide safer nipple preservation to patients with breast cancer located close to the nipple-areolar complex.
Keywords: Breast cancer, Nipple-areolar complex, Nipple base resection, Nipple preservation
INTRODUCTION
Breast cancer is the leading cause of solid malignancy in many developed countries. Compared to other solid malignancies such as lung cancer, gastric cancer, colorectal cancer and pancreatic cancer, vast majority of breast cancers fortunately have been found and diagnosed in an operable condition. Total mastectomy, therefore, has long played an important role in the treatment of breast cancer since the Hasted era and has cured many breast cancer patients [1].
In addition to total mastectomy, breast-conserving therapy emerged in the late 1970s and has greatly contributed to the well-being of breast cancer patients. Two major clinical trials showed comparative overall survival between conventional mastectomy and breast-conserving-therapy in 2002 [2,3]. After that, breast-conserving therapy has become one of the standard surgical options for the treatment of early breast cancer. More breast cancer patients, therefore, have come to be treated with breast-conserving therapy than total mastectomy since 2003.
Tumor size is the most important determinant for the application of breast-conserving therapy. In many countries, exclusion criteria for breast-conserving-therapy generally consist of larger tumor size, multiple cancers in different breast quadrants, extensive intra-ductal spread, patients contraindicated for adjuvant radiotherapy, and no patient’s preference for breast conservation. Besides these factors, breast surgeons have often treated breast cancers located close to the nipple-areolar complex not with breast-conserving therapy but with total mastectomy.
We herein report a case of breast cancer located very close to the nipple-areolar complex successfully treated with breast-conserving surgery using our nipple base resection techniques for complete removal of the sub- and partially intra-nipple mammary gland.
CASE PRESENTATION
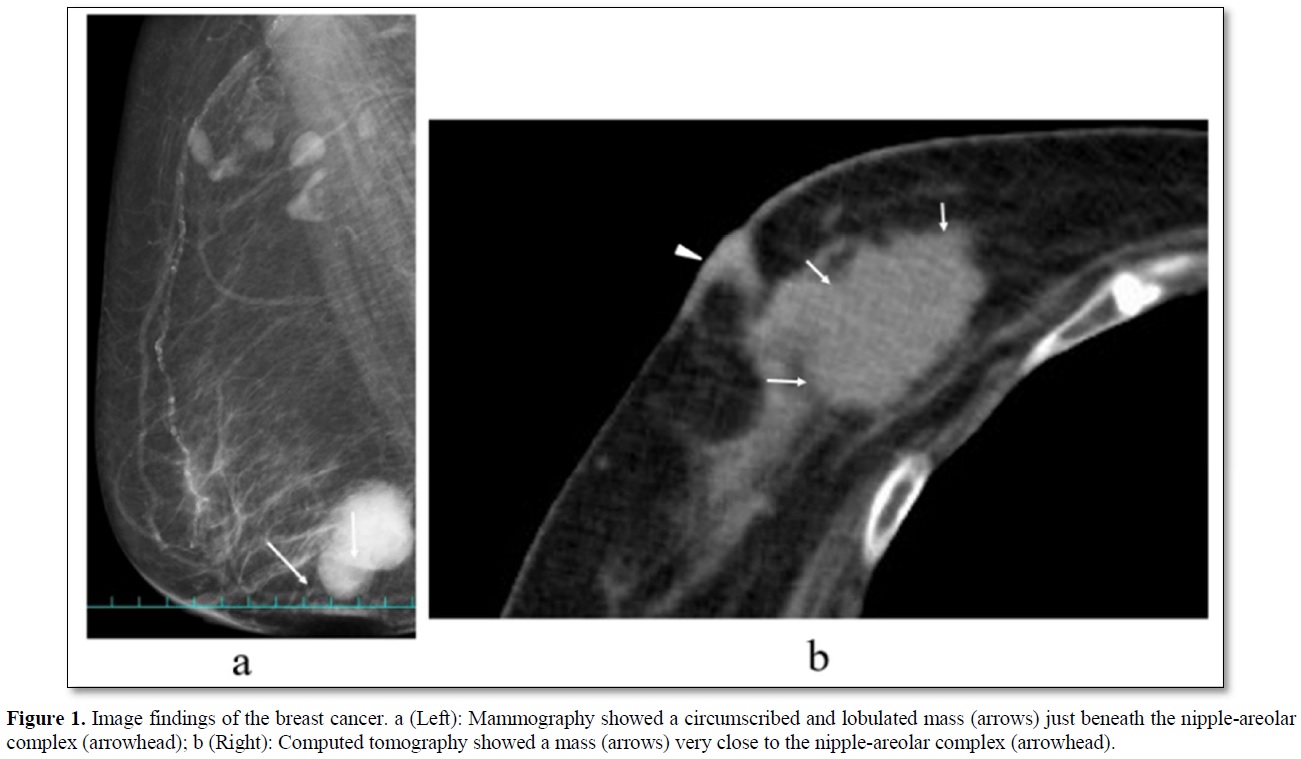
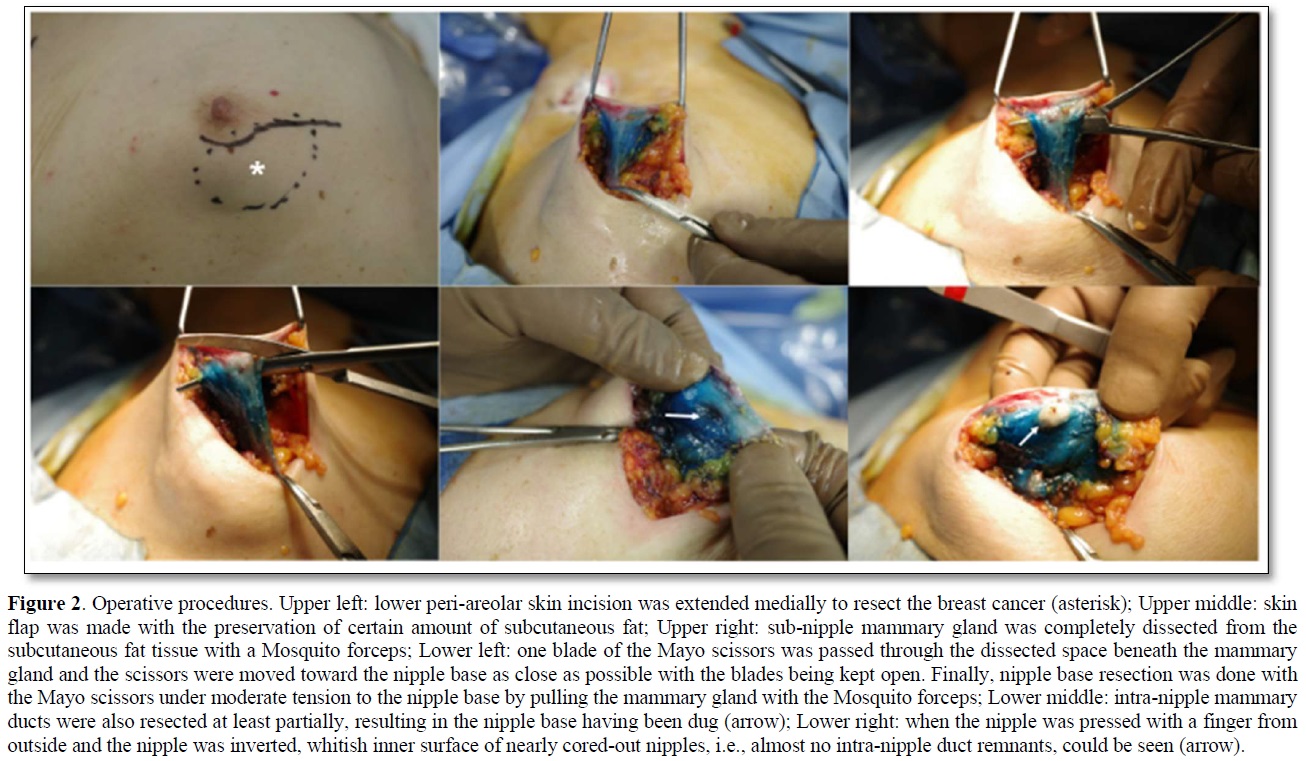
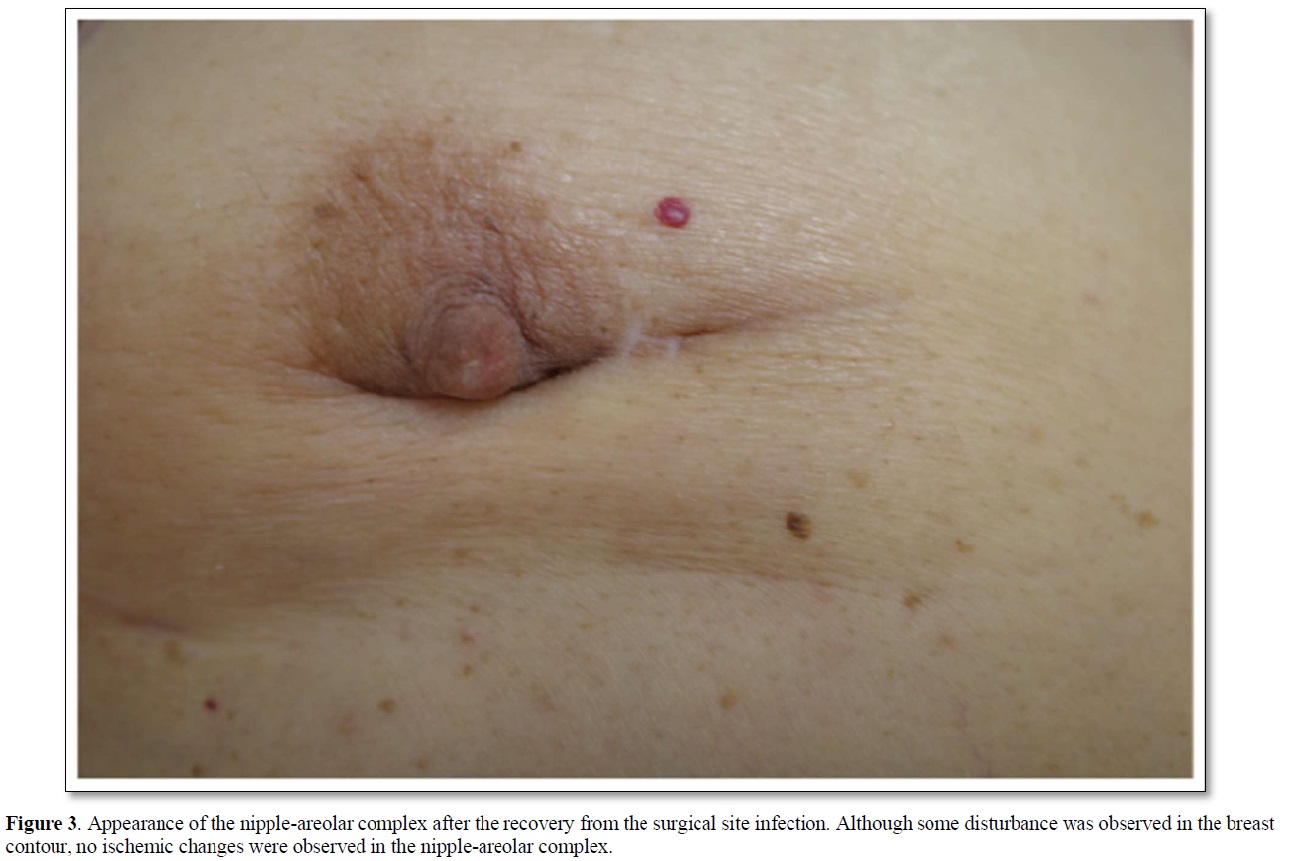
An 84-year-old woman with diabetes mellitus (DM) had noticed a breast tumor in the inner and lower quadrant of the right breast for one year. The patient had undergone various surgeries such as coronary artery bypass grafting, aortic valve replacement, and pacemaker implantation in the left chest wall. Mammography showed circumscribed and lobulated mass just beneath the nipple-areolar complex (Figure 1a). Ultrasound showed an oval mass 4.8c m in size with mixed high and low internal echoes and enhanced posterior echoes. Plain computed tomography (CT) showed the tumor located very close to the nipple-areolar complex (Figure 1b). Aspiration biopsy cytology was done to the tumor and showed atypical cells, highly suggesting invasive carcinoma. We could not evaluate the tumor distribution in detail neither with magnetic resonance imaging nor with enhanced CT due both to the presence of pacemaker in the left chest wall and impaired renal function. Despite the proximity of the breast cancer to the nipple-areolar complex, based on the patient's strong request for nipple preservation, we tried to do partial mastectomy with the preservation of nipple-areolar complex to the patient. Operative procedures were as follows. First, we set a medial horizontal skin incisional line adjacent to the right areola and connected it to a lower semicircular peri-areolar incisional line. Second, we created a skin flap with preserving a certain amount of subcutaneous fat tissue to the flap to ensure venous return around to the nipple-areolar complex. Second, when the flap was almost made to the nipple base with an electrosurgical knife, we bluntly dissected the mammary gland from the subcutaneous fat tissue with a curved Mosquito forceps along with the mammary gland tissue at the lactiferous sinus level. These procedures were initiated at one side of the mammary gland followed by the other side. After enough blunt dissection of the mammary gland on both sides, dissected spaces were connected to each other. Then, one blade of the Mayo scissors was passed through the dissected space beneath the mammary gland and moved the scissors themselves toward the nipple base as close as possible with the blades being kept open. Next, after confirming no excess tissue pinched between the blades of the Mayo scissors, we cut the sub-nipple mammary gland with adequate counter traction to the nipple base by pulling the mammary gland towards the opposite side of the nipple. Finally, we confirmed the nipple base status with no visible sub-nipple mammary gland left on the nipple base followed by pathological confirmation of negative surgical margins in all directions including nipple base on frozen section (Figure 2). The cavity after partial mastectomy, therefore, was sutured without drain insertion to it. Due to the positivity of sentinel node biopsy, lymph node dissection was added to the axilla with drain insertion. Postoperative pathological study showed no positive surgical margins and atypical cells growing in a tubule-forming fashion in the mucous lake, leading to the final diagnosis of mucinous carcinoma. The patient had a high lymph outflow from the axilla, requiring about 2 weeks to be discharged. The patient, however, was re-admitted to the hospital due presumably to DM-induced surgical site infection but showed favorable wound healing without any nipple necrosis (Figure 3). Due to her old age, the patient received neither chemotherapy nor radiotherapy to the conserved breast and has been well without recurrence for nine months.
DISCUSSION
In general, small breast cancers are good candidates for breast-conserving therapy. Various factors, however, can affect cosmetic outcome among patients with breast cancer indicated for breast conservation. The larger the tumor size, the worse the cosmetic outcome after partial mastectomy. In addition, not only the size of the tumor but also the size of the breast has a great impact on cosmetic outcome. Breast-conserving therapy, therefore, is indicated for breast cancer 3cm or smaller in Japanese patients [4] and for that up to 5cm in American patients [5].
Besides lymphatic permeation and hematogenous spread, breast cancer can widely spread in the breast in a stromal invasion fashion and/or a ductal spread fashion. Even small breast cancers, therefore, have been treated not with breast-conserving therapy but with total mastectomy due to the possible cancer, especially non-invasive ductal cancer, spread mainly toward the nipple-areolar complex. Improvement of spatial resolution of the magnetic resonance imaging (MRI) has made us more accurately evaluate the stromal invasion to the nipple-areolar complex preoperatively than the era without MRI evaluation on one hand [6]. No image modalities including MRI can detect small amount of non-invasive ductal spread to the nipple on the other hand. Unexpected positive margins on frozen section, therefore, sometimes force us to treat the patients not with nipple preservation but with nipple resection, or even with total mastectomy [7]. In this case, we could not precisely evaluate the cancer spread in the breast preoperatively due both to the presence of pacemaker and her renal impairment. We, therefore, intended to resect the possible intra-ductal spread, even if present, between the main tumor and the nipple.
Many surgeons undoubtedly believe that ischemia of the nipple due to full mammary gland resection could easily cause nipple necrosis. Breast surgeons unfamiliar with nipple base resection hence often resect the nipple base in such a manner to leave certain amount of mammary gland under the nipple. Rusby [8] however, reported that one-third of the blood supply to the nipple was from the mammary gland and the other two-thirds were from the skin. These findings highly suggest safer nipple preservation, even if the mammary gland is completely removed, as long as the blood flow in the nipple skin is maintained.
It is well known that non-invasive ductal cancers spread more widely in the breast than small invasive ductal cancers, leading to less application rate of breast conservation to stage 0 ductal cancers than to stage 1 ductal cancers. This phenomenon is further amplified by the idea that even slight involvement of non-invasive ductal cancer just around the nipple should not be the candidates for nipple preservation. On the other hand, non-invasive ductal cancer does not invade the nipple skin except for the parietal part of the nipple. These facts strongly encourage breast surgeons to resect non-invasive ductal component spreading almost to the nipple or even slightly into the nipple with some surgical intervention, i.e., nipple incision or partial nipple resection, to the nipple-areolar complex. Our nipple base resection techniques, however, can provide another alternative, i.e., feasible resection of ductal spreading for the nipple direction without direct surgical intervention to the nipple-areolar complex, for patients with breast cancer located very close to the nipple-areolar complex.
The main points of our nipple base resection technique are as follows. First, certain amount of sub-areolar fat tissue should be preserved to prevent inhibition of venous return from the nipple, i.e., nipple congestion. In order to ensure the safety of preserving the subcutaneous fat around the nipple-areolar complex, it is important for breast surgeons to select the patients suitable for nipple and subcutaneous fat preservation. Therefore, breast cancers with massive lymphatic permeation should be contra-indicated for our surgical procedures and be treated not with primary surgery but with primary chemotherapy followed by some surgery using our nipple base resection techniques, when observed marked response to the primary chemotherapy [9]. Second, breast surgeons should detach the nib-nipple mammary gland from surrounding fat tissue with the curved Mosquito forceps. Because the horizontal cross-section of the sub-nipple mammary gland is oval shape, this blunt dissection procedures are easier with the curved forceps than with the straight forceps. Then, appropriate counter-traction for nipple base enables surgeons to also resect some part of the intra-nipple mammary gland. In addition, our operative techniques safely and uniformly enable breast surgeons to prevent skin damage on sub-nipple mammary gland resection by passing one blade of the scissors into the dissected space just beneath the sub-nipple mammary gland and moving the blades toward the nipple base with the blades being kept open.
Our surgical techniques allow many breast surgeons, including inexperienced young surgeons, to safely and easily excise the intraductal breast cancer spread toward the nipple-areolar complex without major nipple complication. This report mainly mentioned our surgical techniques how to safely preserve the nipple-areolar complex for a breast cancer located close to the nipple with limited post-operative follow-up. Not only preoperative nipple complication but also long-term local control of our surgical techniques should be evaluated in the near future.
In conclusion, our nipple base resection techniques can offer feasible nipple base resection for breast cancer located close to the nipple-areolar complex. We are convinced that the acquisition of our surgical techniques will greatly contribute to the increase in the number of patients who will benefit from nipple preservation.
STATEMENT OF ETHICS
The study was approved by the Kishiwada Tokushukai Hospital Ethics Committee (IRB #Case 20-06). Written informed consent was obtained from the patient for the publication of this case report and any accompanying images.
CONFLICT OF INTEREST STATE
The authors have no conflicts of interest to declare.
FUNDING SOURCES
No funding was received for this research.
AUTHOR CONTRIBUTIONS
Naoko Abe contributed to the design of the report. Shoji Oura drafted the manuscript.
Shinichiro Makimoto revised the manuscript. All authors have read and approved the final version of the manuscript.
DATA AVAILABILITY STATEMENT
All data generated during this study are included in this article. Further inquiries can be directed to the corresponding author.
REFERENCES
No Files Found
Share Your Publication :